Prof. Hongli Hu’s Research on Structures of G-protein-coupled Receptors Published in Nature
Professor Hongli Hu from the School of Life and Health Sciences, The Chinese University of Hong Kong, Shenzhen recently published an article entitled "Structure of the M2 Muscarinic Receptor-β-arrestin Complex in a Lipid Nanodisc" in the internationally renowned journal Nature. This paper presents the molecular mechanisms underlying the interaction of β-arrestin with GPCRs reconstituted in lipid nanodiscs, analyzes the key binding areas for their interaction, and provides guidance for the development of new drugs for related diseases.

New research on G-protein-coupled receptor structures published by Prof. Hongli Hu in Nature
The latest research on G-protein-coupled receptors (GPCRs) and the structures of β-arrestin by Professor Hongli Hu of The Chinese University of Hong Kong, Shenzhen was published online in the journal Nature in January 2020. This paper present a cryo-electron microscopy structure of β-arrestin 1 (βarr1) in complex with M2 muscarinic receptor (M2R) reconstituted in lipid nanodiscs, suggests what contribute to β-arrestin’s signaling initiation, and thus provide guidance for drug development against such receptors.
Prof. Hongli Hu is an Assistant Professor and doctoral supervisor at the School of Life and Health Sciences, The Chinese University of Hong Kong, Shenzhen and head of the Independent Research Group at the Kobilka Institute of Innovative Drug Discovery. The M2 muscarinic receptor (M2R) studied in this article plays an important role in the regulation of human heart rate and many central nervous system functions.
The published article "Structure of the M2 muscarinic receptor-β-arrestin complex in a lipid nanodisc" is the result of a collaboration between Professor Hongli Hu and Professor Georgios Skiniotis of Stanford University and Professor Robert J. Lefkowitz of Duke University. Professor Lefkowitz has received the 2012 Nobel Prize in Chemistry for his outstanding contributions to the field of GPCRs.

Structures of β-arrestin and its interaction with GPCRs
Click the link to see the article
https://www.nature.com/articles/s41586-020-1954-0
About the Author

Assistant Professor
Doctoral Supervisor
Head of Independent Research Group
Prof. Hongli Hu is an Assistant Professor and doctoral supervisor at the School of Life and Health Sciences, The Chinese University of Hong Kong, Shenzhen and head of the Independent Research Group at the Kobilka Institute of Innovative Drug Discovery. Currently, she is responsible for the study on the structural biology of G-protein-coupled receptor complexes.
She mainly uses Cryo Electron Microscopy to obtain the high-resolution structure of GPCR and studies the interaction between membrane proteins and their regulatory proteins. She has conducted extensive collaborative research with Prof. Brian Kobilka and Prof. Robert Lefkowitz of 2012 Nobel Laureates in chemistry at the University of Michigan and Stanford University. These studies have led to major breakthroughs in the structural study of several important GPCRs in classes A, B, and C. Seven of the scientific results were published in Nature and Cell, and have received wide international attention with 688 citations.
About Nature
First published in 1869, Nature is the world's leading multidisciplinary science journal. Many of the most important and cutting-edge findings in scientific research are published as articles in Nature, which had an impact factor of 41.456 in 2014.
About Research Results
GPCRs are the largest family of membrane proteins and mediate most cellular responses to hormones and neurotransmitters, as well as being responsible for vision, olfaction and taste. GPCRs also interacts with intracellular signaling protein molecules and thus induces various cellular responses.
Currently, more than 800 GPCRs have been found in humans and play an integral role in almost all important physiological activities. It is an important drug target for cardiovascular diseases, neurological diseases, inflammation, metabolic diseases, cancer and other major diseases. Thirty-four percent of the drugs on the market are targeted for GPCRs to act, and studying GPCRs has great significance for drug development for these diseases.
The GPCR family is very diverse and functions in a very important way in the human body. Professor Hu's team uses cryo-electron microscopy to study the structure of GPCRs molecules and understand the mechanisms of their signaling, thereby providing guidance for the development of new drugs for related diseases.
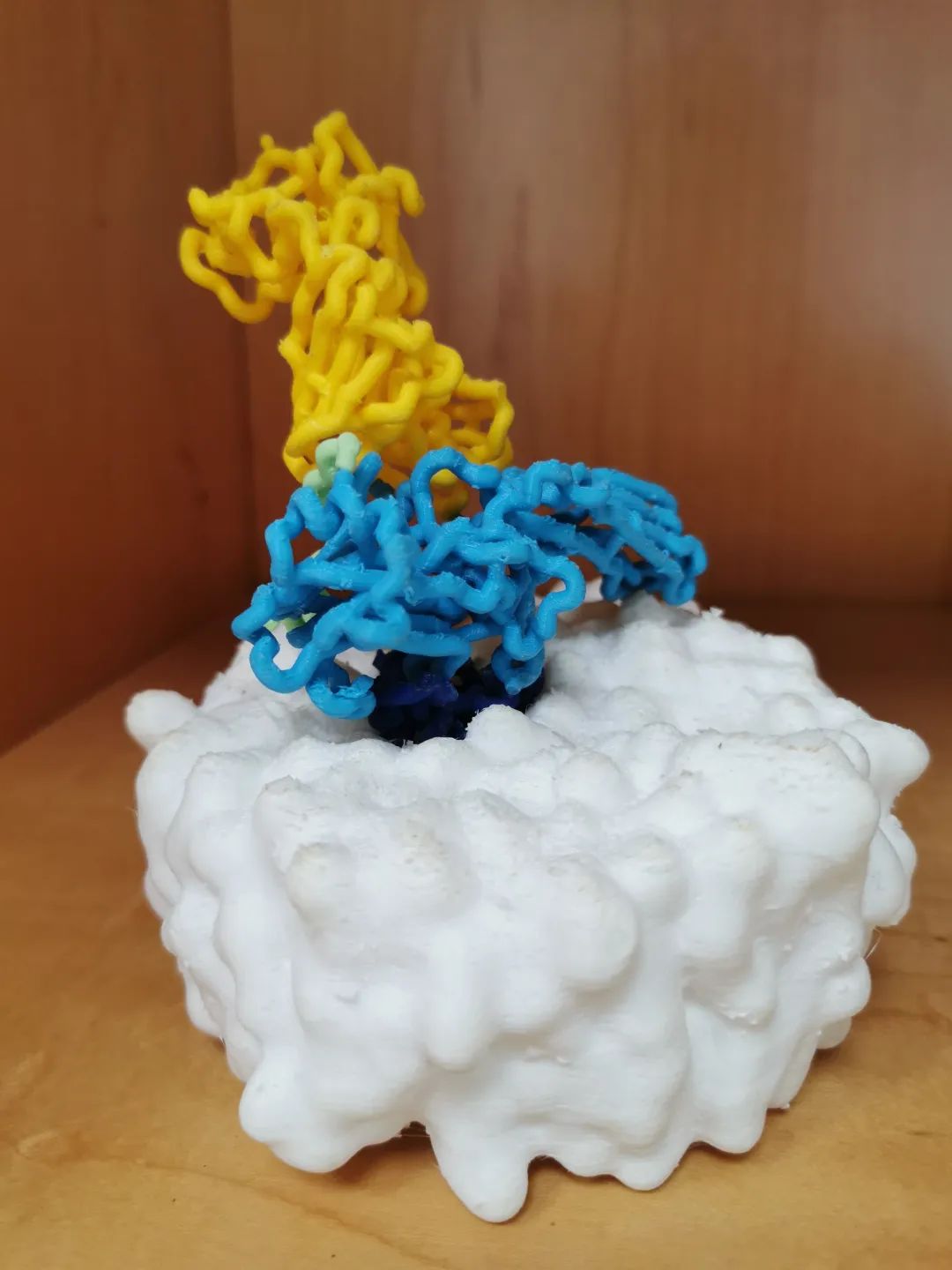
3D printed model of GPCR/β-arrestin
Professor Hu said: "We can liken GPCR to a communication chamber on the cell membrane. After a photon or odor molecule is picked up by it, it has to pass the information inside the cell for the cell to respond. GPCR primarily transmits signals through G-proteins and β-arrestins, which tend to induce different cellular responses."
Leading international GPCR researchers have published research over the past few years on the structure of multiple GPCR interactions with G-proteins, but haven't fully unravelled how GPCRs recruit β-arrestins.
Prof. Hu has studied the interaction of GPCRs with β-arrestins reconstituted in lipid nanodiscs and analyzed the key binding sites of the interaction. The GPCR she focuses on in her article is M2R, which plays an important role in regulating human heart rate and many central nervous system functions. This article demonstrates the recruitment of β-arrestins to this receptor, helps to understand the initiation mechanism of β-arrestins signaling, and provides guidance for drug development against such receptors.




